Abstract
Patients (pts) with cancer are at increased risk for morbidity and mortality from SARS-CoV-2 infection (COVID-19), which is at least partly attributable to their relatively advanced age and higher comorbidity burden. Pts with hematologic malignancies are at particularly high risk for adverse outcomes from COVID-19 due to immune impairment from the underlying disease and the impact of therapies that disable innate, B cell, and T cell immunity. mRNA vaccines against SARS-CoV-2 (BNT162b2 and mRNA-1273) are effective in preventing COVID-19, reducing viral transmission, and dramatically reducing the risk of COVID-19-related hospitalization and mortality in immunocompetent persons. Pts with various types of active cancer, and receiving different types of therapy, were underrepresented in COVID-19 vaccine trials, hence large observational studies have been instrumental in elucidating the spectrum of early vaccine response in these pts. In our previously published work (Ghione et al, Blood 2021), we observed severely impaired antibody responses to the primary vaccination series, which consisted of 2 administrations of mRNA COVID-19 vaccines in pts with lymphoma receiving B cell-depleting therapies (BCDT). Importantly, impaired antibody responses to vaccination persisted until 9 months after completion of rituximab (anti-CD20). This and other work that revealed impaired vaccine-induced immunity in pts with cancer influenced policy at a national and international level, including the current recommendations for booster vaccines and the CDC guidance regarding prophylactic monoclonal antibodies.
Despite these recommendations for prophylactic clinical management, a key gap in the field is validated immunologic metrics that predict vaccine-induced protection from severe COVID-19. We hypothesized that pts on active therapy for lymphoma, including those on BCDTs, will mount T cell responses despite impaired serologic responses to repeated mRNA COVID-19 vaccination.
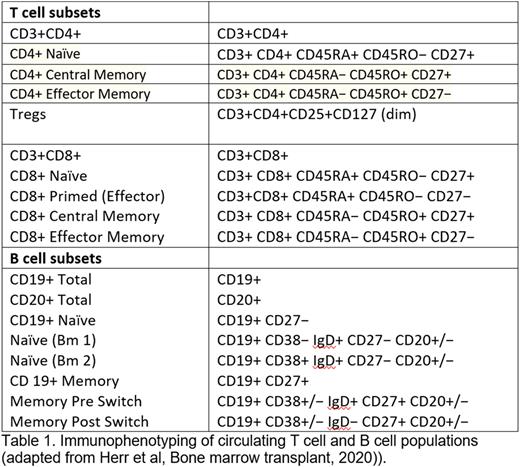
To test this hypothesis, we have obtained samples from pts with lymphoma using an open and enrolling prospective protocol to collect serial peripheral blood samples following primary and booster SARS-CoV-2 vaccination in pts with cancer. To date, 250 samples from 98 pts with lymphoma (NHL, n=67 and CLL, n=31) have been collected of which 75 had received/were receiving BCDT and 23 were either on observation or were on a treatment not including BCDT. Only 26/75 (34%) pts on treatment/previously treated with BCDT mounted a significant IgG Ab response, as opposed to 22/23 (91.6%) pts who were not on BCDT (p= 0.007). We have analyzed these pts for T-cell response to SARS-CoV-2 vaccination following initial and booster vaccination using the T-Detect COVID Test (Adaptive Technologies). This assay combines multiplex PCR and next-generation sequencing (NGS) to quantify rearranged T-cell receptor beta (TCR β) gene sequences specific for SARS-CoV-2. Flow cytometry for phenotypic analysis of T and B cell populations was performed at serial time points. (Table 1). This data is currently being analyzed and we will report our findings at the meeting.
Our comprehensive analysis will provide a detailed picture of how treatment with BCDTs impacts T cell clonal expansion and fitness in pts with lymphoma and elucidate the interplay between B and T-cell responses to SARS-CoV-2 vaccination. This work will help refine our ability to risk-stratify these pts. Moreover, it will serve as a framework for analyzing data sets in future studies aimed at assessing T cell and humoral responses as immune correlates of vaccine-induced protection from COVID-19 (incidence and severity) and other vaccines, in pts with lymphoma, and other diseases treated with BCDT (such as Multiple Sclerosis).
Disclosures
Goel:NextCure: Membership on an entity's Board of Directors or advisory committees, Research Funding; Apellis: Research Funding. Griffiths:Takeda Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Picnic Health: Honoraria; Medicom Worldwide: Honoraria; Celldex Therapeutics: Research Funding; Blueprint Medicines: Research Funding; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex Pharmaceuticals: Research Funding; Apellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Physician Educational Resource: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AAMDSIF: Honoraria. Torka:Genentech: Consultancy; ADC Therapeutics: Consultancy; TG Therapeutics: Consultancy; Lilly USA: Consultancy; Epizyme: Consultancy; Targeted Oncology, Physician Education Review: Honoraria. Ghione:Secura Bio: Consultancy; Kite Pharma: Research Funding; Kyowa Hakko Kirin: Consultancy; AstraZeneca Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal